
Astaxanthin and eye Fatigue
The advances of information technology, software and electronics gave rise to the widespread use of screen based equipment or visual display terminals (VDT) especially at work or leisure. VDT’s are important because it forms the essential interface between an operator and computer to perform specific tasks. The problem acknowledged by the ophthalmic community is that habitual users of VDTs often lead to higher visual fatigue complaints. This phenomenon known as asthenopia prompted a large number of occupational safety studies. For example, epidemiological studies over the last decade revealed significant factors that contribute to eye fatigue. These studies, sometimes involving up to 6,000 sufferers identified the following causes: insufficient lighting, poor ergonomics and uncorrected vision. Despite the new information, follow-up studies later showed that the implemented improvements were only effective in 50% of suffers.
The possible explanations for this observation could be that other factors remained undiscovered, poor implementation of improvements, or visual work had become even more visually demanding. It is likely to be a combination of these factors so that current solutions are insufficient to reduce asthenopia.

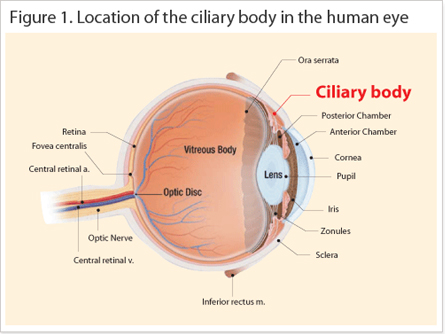
The symptoms of asthenopia caused include sensitivity to glare, headaches, sore eyes, and blurred vision. Standardized questionnaires that assessed subjective eye fatigue symptoms are in most cases mild, but symptoms get progressively worse if the causes are not rectified. Furthermore, certain ophthalmological tests can also detect eye problems, for example accommodation amplitudes, rate of accommodative reaction (positive and negative directions), critical flicker fusion (CFF) and pattern visual evoked potential (PVEP). So far, nine Japanese clinical studies conducted by six independent ophthalmological establishments were able to conclude the efficacy of astaxanthin to alleviate visual asthenopia by observed improvements in the accommodation function and recovery of the ciliary body (Figure 1); retinal blood flow and
Asthenopia
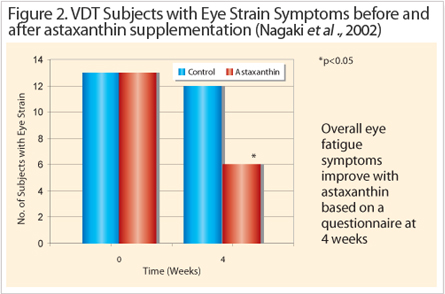
Asthenopia otherwise called eye fatigue occurs on a daily cycle, in that the visual performance generally decreases naturally from morning until night. This problem exacerbates with a daily VDT load that lasts between 4 to 7 hours by affecting the accommodation performance of the ciliary body, which controls lens refraction. A couple of randomized double blind placebo controlled pilot studies demonstrated the positive effects of astaxanthin supplementation on visual function. For example, a study by Nagaki et al., (2002), demonstrated that subjects (n=13) who received 5 mg astaxanthin per day for one month showed a 54% reduction of eye fatigue complaints (Figure 2). In a sports vision study led by Sawaki et al., they demonstrated that depth perception and critical flicker fusion had improved by 46% and 5% respectively on a daily dose of 6 mg (n=9). The effect of astaxanthin on visual performance prompted a number of other clinical studies to evaluate the optimum dose and identify the mechanism of action.
Reducing Asthenopia
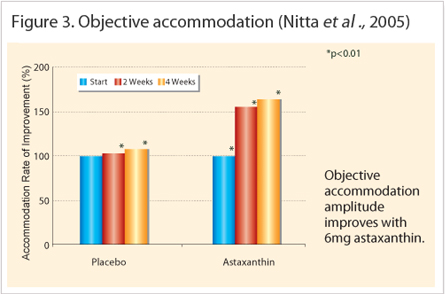
Reducing Asthenopia study by Nakamura (2004), demonstrated significant improvements in reducing asthenopia and positive accommodation for the 4 mg (p<0.05) and 12 mg (p<0.01) groups. However, it was not until Nitta et al., (2005), who established the optimum daily dose at 6 mg (n=10) for a period of 4 weeks by comparing eye fatigue using a visual analogue scale (VAS) based questionnaire and accommodation values. Overall, the 6 mg group improved significantly better at week 2 and 4 of the test period. Furthermore, results obtained by Shiratori et al., (2005) and Nagaki et al., (2006), also confirmed the previous findings that astaxanthin supplementation at 6 mg for 4 weeks improved symptoms associated with tiredness, soreness, dryness and blurry vision. Another study by Takahashi & Kajita (2005), also demonstrated that astaxanthin attenuates induced-eye fatigue, as opposed to treating eye fatigue, which suggests prevention rather than treatment. Astaxanthin treated groups (asthenopia negative) were able to recover quicker than the control group after heavy visual stimulus Since the questionnaires may be subjectively limited, the direct measurements of parameters associated with asthenopia are better indicators. These include accommodation amplitude (Figure 3); rate of accommodation reaction (positive and negative directions); CFF (visual central nervous system activity) and PVEP (ophthalmic neural conductivity). Based on the information available, the accommodation related measurements consistently improved after the treatment period (Nagaki et al., 2002, 2006; Nakamura et al., 2004; Takahashi & Kajita, 2005; Shiratori et al., 2005; Nitta et al., 2005) whereas the CFF and PVEP remained inconclusive (Sawaki et al., 2002; Nagaki et al., 2002; Nakamura et al., 2004). Therefore, the mechanism by which astaxanthin improved eye fatigue strongly indicates accommodation
Mechanism of Action : Improved Accommodation, Increased Blood-flow and Anti –inflammation Accommodation measures the lens refractive property and it corresponds to the ciliary body function. This small ocular muscle controls the lens thickness in order to focus the light on the retina. In heavy visual workloads, the eye is focuses on a fixed object distance for extended periods that will cause muscle spasms or develop fatigue detectable by accommodation tests. These tests are interrelated and include the following: accommodation amplitude; accommodation reaction (positive or negative) and high frequency component (HFC). Each clinical study used a combination of accommodation tests to indicate the amount of fatigue present. For example, increased accommodation amplitude in all treated subjects indicated improved reaction on near and far objects (Nagaki et al., 2002, 2006; Nakamura et al., 2004). Figure 4 and Figure 5 reveals the higher rate of accommodation reactions measured in astaxanthin treated groups.
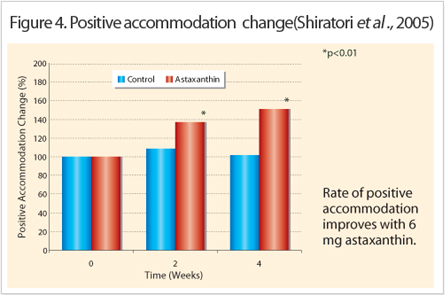
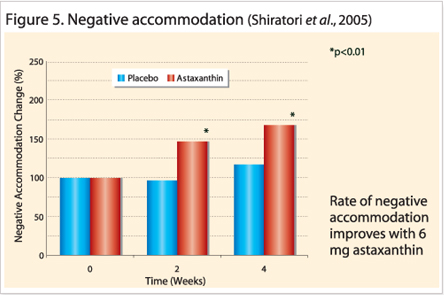
These indicate the speed at which the ciliary body reacted to the direction change of focus (positive means from a near object at 35 centimeters to distant object at 5 meters or vice versa); (Nitta et al., 2005; Shiratori et al., 2005; Nakamura et al., 2005).
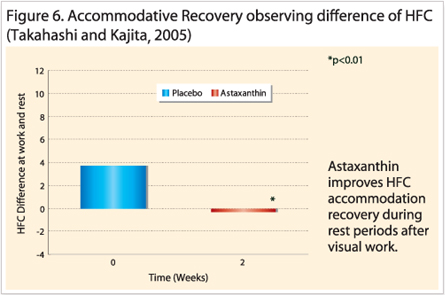
The effects of astaxanthin are significant from 2 weeks. Another technique called HFC directly measured the microfluctuations in the lens during the accommodation response and typical values exist between 50 and 60 for normal eyes. Asthenopia sufferers (values greater than 60) experienced faster rates of recovery (Figure 6) in that their HFC results decrease towards normal values in less time compared to control groups (Takahashi & Kajita, 2005).
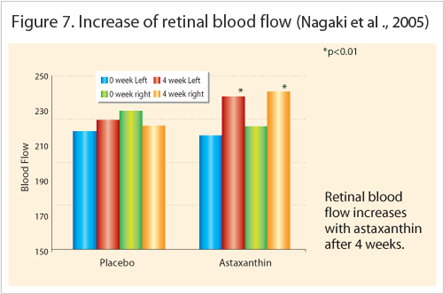
Another randomized placebo controlled study by Nagaki et al., (2005) detected the increase of retinal blood flow in the astaxanthin treated group that received 6 mg for 4 weeks (n=14, p<0.01). Even though the precise reason for accommodation improvement seen with astaxanthin is not yet clear, the author postulated that based on the rheological improvement measured in the retinal capillary vessels, most likely means more blood also reaches the ciliary body and provides more nourishment to the ciliary muscles. Furthermore, the rheological improvement agreed with Nagaki et al., (2005) who studied ten healthy subjects treated with 6 mg astaxanthin for ten days (Figure 7). The blood exhibited significantly higher flow rates (ex-vivo) compared to the control group (p<0.05) utilizing the micro-array channel flow analyzer (MC-FAN).
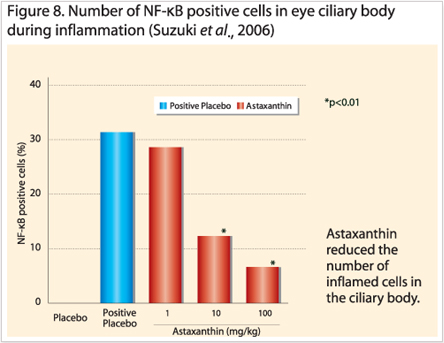
Lastly, a top Japanese ophthalmology research collaboration between Hokkaido, Yokohama and Tokyo concluded anti-inflammatory properties of astaxanthin in endotoxin-induced uveitis (EIU or eye inflammation) both in vivo and in vitro models. Ohgami et al., (2003) observed in a dose dependant fashion that astaxanthin doses of 1, 10 or 100mg/kg dose in rats (n=8, p<0.01) significantly reduced inflammation markers such as nitric oxide synthase (NOS), prostaglandin E2 (PGE2) and tumor necrosis factor (TNF)-α. Other reduced biomarkers were cellular infiltration and protein build up in the aqueous humor.
For astaxanthin to work, it has to pass through the human blood-retinal barrier (BRB) for which there is no direct evidence because a non-invasive specific quantification method is not available. However, the BRB is a selective barrier similar to the blood-brain barrier (BBB) which is better understood, so astaxanthin is expected to pass through because of the molecular size is less than 600 Daltons. Furthermore, astaxanthin belongs to the same carotenoid xanthophyll group as lutein and zeaxanthin, both of which are concentrated in the macular region of the eye. Unlike, beta-carotene or lycopene (carotenes), xanthophylls are the only carotenoids detected in the eye so far.
Outlook
References
1. Iwasaki & Tawara, (2006). Effects of Astaxanthin on Eyestrain Induced by Accommodative Dysfunction. Journal of Eye (Atarashii Ganka) (6):829-834.
2. Suzuki et al., (2006). Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kB signaling pathway. Exp. Eye Res., 82:275-281.
3. Nagaki et al., (2006). The supplementation effect of astaxanthin on accommodation and asthenopia. J. Clin. Therap. Med., 22(1):41-54.
4. Miyawaki et al., (2005). Effects of astaxanthin on human blood rheology. J. Clin. Therap. Med., 21(4):421-429.
5. Nitta et al. (2005). Effects of astaxanthin on accommodation and asthenopia-Dose finding study in healthy volunteers. J. Clin. Therap. Med., 21(6):637-650.
6. Shiratori et al. (2005). Effect of astaxanthin on accommodation and asthenopia- Efficacy identification study in healthy volunteers. J. Clin. Therap. Med., 21(5):543-556.
7. Takahashi & Kajita (2005). Effects of astaxanthin on accommodative recovery. J. Clin. Therap. Med., 21(4):431-436.
8. Nagaki et al. (2005). The effects of astaxanthin on retinal capillary blood flow in normal volunteers. J. Clin. Therap. Med., 21(5):537-542.
9. Nakamura et al l. (2004). Changes in Visual Function Following Peroral Astaxanthin. Japan J. Clin. Opthal., 58(6):1051-1054.
10. Ohgami et al., (2003). Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthal. Vis. Sci., 44(6):2694-2701.
11. Nagaki Y., et al., (2002). Effects of astaxanthin on accommodation, critical flicker fusions, and pattern evoked potential in visual display terminal workers. J. Trad. Med., 19(5):170-173.
12. Sawaki, K. et al. (2002) Sports performance benefits from taking natural astaxanthin characterized by visual activity and muscle fatigue improvements in humans. J. Clin. Ther. Med., 18(9):73-88.





